Anemia of Chronic Disease: Diagnosis, Mechanisms, and Management (USMLE High‑Yield)
Why this matters
Anemia of chronic disease (ACD)—also called anemia of inflammation—is one of those topics that quietly appears across organ systems and test blocks. It mimics iron deficiency anemia (IDA) yet responds very differently to therapy. On the wards, missing ACD leads to unnecessary iron studies, undertreatment of the driver (inflammation/CKD), and persistent symptoms. On exams, success hinges on recognizing the hepcidin story and reading iron studies in context.
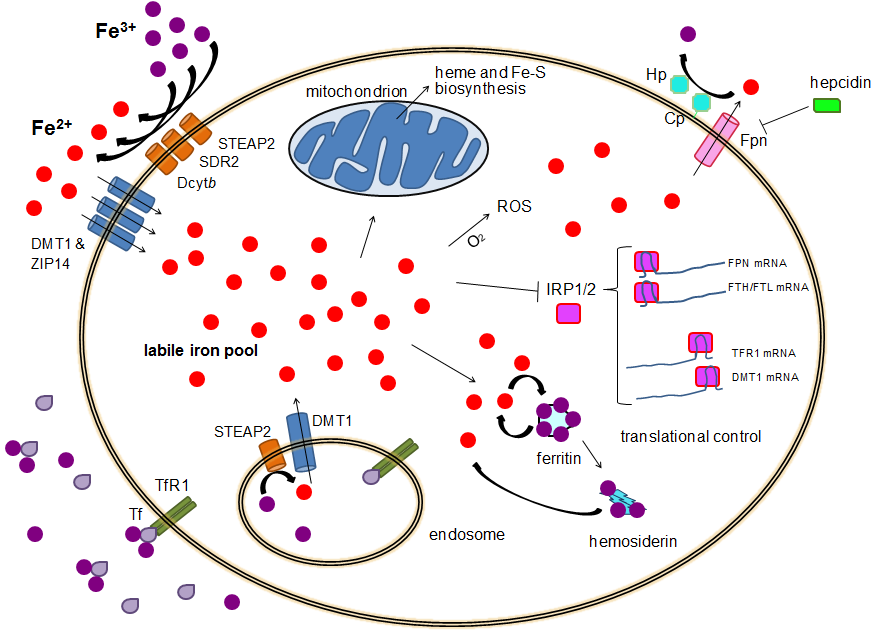
Step 1 integration: Interleukin‑6 from chronic inflammation upregulates hepcidin in the liver. Hepcidin binds and triggers internalization of ferroportin, the iron exporter on enterocytes and macrophages, reducing dietary iron absorption and trapping recycled iron inside the reticuloendothelial system.
Anemia of Chronic Disease Pathophysiology at a glance
- Cytokine milieu: IL‑6 (and others) raise hepcidin → ↓ ferroportin → iron sequestration.
- Blunted EPO response: Inflammation and CKD suppress renal erythropoietin production and bone‑marrow sensitivity to EPO.
- Shortened RBC survival: Mild hemolysis‑like features from inflammatory stress.
- Net effect: Adequate or high iron stores (ferritin) but poor availability (low serum iron), leading to normocytic or mildly microcytic anemia.
Step 1 integration: Ferritin is an acute‑phase reactant—inflammation increases ferritin independent of iron quantity. That’s why ferritin may be “normal/high” in ACD despite functional iron starvation.
Vignette #1 — RA clinic “iron deficiency”
A 52‑year‑old man with long‑standing rheumatoid arthritis reports progressive fatigue. Exam is non‑focal. CBC: Hb 10.2 g/dL, MCV 85 fL. Iron studies: ferritin 250 ng/mL (↑), serum iron ↓, TIBC ↓, transferrin saturation ↓.
Question: How should we treat him? Iron pills?
Answer: This is ACD. High ferritin with low TIBC is the giveaway—iron is present but inaccessible. Treat inflammation and consider EPO support if appropriate.
Step 1 integration: High ferritin + low TIBC = classic ACD pattern due to hepcidin‑mediated ferroportin blockade. In IDA, ferritin is low and TIBC is high.
Distinguishing ACD from iron deficiency anemia (IDA)
| Feature | ACD | IDA |
|---|---|---|
| Ferritin | Normal/↑ (acute‑phase) | ↓ |
| Serum iron | ↓ | ↓ |
| TIBC | ↓ (liver produces less transferrin in inflammation) | ↑ |
| Transferrin saturation | ↓ | ↓ (often lower than ACD) |
| MCV | Normocytic or mildly microcytic | Microcytic |
| RDW | Normal or mildly ↑ | Often ↑ |
| Bone marrow | Iron present in macrophages | Depleted iron |
Practical pearl: If ferritin is <30 ng/mL, think true iron deficiency even in inflammatory states; if ferritin is >100 ng/mL with low TIBC, ACD is more likely. Grey zones need clinical judgment.
Step 1 integration: Hepcidin’s blockade of enterocyte iron export explains why oral iron is often ineffective in pure ACD—absorption is throttled.
Where you’ll encounter ACD
- Autoimmune disease: RA, SLE, IBD.
- Infections: TB, HIV, osteomyelitis, endocarditis.
- Malignancy: Solid tumors and hematologic cancers.
- Chronic kidney disease: Reduced EPO + inflammation → potent ACD driver.
Step 1 integration: In CKD, low EPO is foundational; uremic toxins and inflammation elevate hepcidin, compounding iron‑restricted erythropoiesis.
Vignette #2 — CKD patient with “normal ferritin”
A 65‑year‑old woman with stage 4 CKD presents with exertional dyspnea. Hb 9.6 g/dL, MCV 88 fL. Ferritin 180 ng/mL, serum iron low, TIBC low, transferrin saturation 16%.
Interpretation: CKD‑related ACD. Ferritin looks reassuring but reflects inflammation and adequate stores, not availability.
Next steps: Address CKD anemia with erythropoiesis‑stimulating agents (ESAs) when indicated; evaluate iron status (TSAT/ferritin) and consider IV iron if absolute deficiency coexists or TSAT is low despite moderate ferritin.
Step 1 integration: ESAs (epoetin alfa, darbepoetin) stimulate erythroid progenitors if iron is available—pair with iron repletion only when deficient.
Workup workflow
- Confirm anemia (Hb below sex‑specific reference).
- Look at MCV/RDW (normocytic ± mild microcytosis; RDW not dramatically elevated in pure ACD).
- Order iron studies: ferritin, serum iron, TIBC, transferrin saturation.
- Assess inflammation/CKD/malignancy (ESR/CRP, renal function, clinical context).
- Consider mixed anemia (ACD + IDA is common—e.g., RA patient with GI bleed from NSAIDs).
Tip: Soluble transferrin receptor rises in IDA but is typically normal in pure ACD, helping in ambiguous cases (not always on exam, but good to know).
Management principles
- Treat the underlying driver (control autoimmune disease, treat infection, manage malignancy). Without dampening inflammation, iron won’t mobilize.
- CKD anemia: Consider ESAs; monitor targets and blood pressure. Ensure adequate iron availability—IV iron may be used if absolute deficiency or suboptimal TSAT.
- Iron therapy: Reserve for proven iron deficiency or mixed pictures. Oral iron often underperforms in ACD due to hepcidin—IV iron is sometimes favored when deficiency is documented or absorption is limited.
- Transfusion: For symptomatic severe anemia or hemodynamic instability—short‑term bridge only.
Step 1 integration: ESAs can increase blood viscosity and hypertension risk; iron overload is a concern with indiscriminate IV iron—track ferritin/TSAT.
Pitfalls and test‑day traps
- Treating every anemia with iron. In ACD, ferritin is normal/high; iron won’t fix the fundamental hepcidin block.
- Ignoring CKD. A “mysterious normocytic anemia” in an older adult with low GFR is CKD until proven otherwise.
- Overlooking mixed etiologies. An ACD pattern can mask concurrent GI blood loss; repeat iron studies after inflammation improves or evaluate stool/EGD when indicated.
- Misreading ferritin. Remember it’s an acute‑phase reactant—interpret alongside TIBC and the clinical picture.
Step 1 integration: Pair patterns with mechanisms: high ferritin + low TIBC → inflammatory signal; low ferritin + high TIBC → true iron depletion.
Quick compare: ACD vs thalassemia vs sideroblastic (exam‑savvy add‑on)
| Feature | ACD | Thalassemia trait | Sideroblastic |
|---|---|---|---|
| MCV | N or mildly ↓ | Often markedly ↓ | N or ↓ |
| RDW | Normal/mild ↑ | Normal | ↑ |
| Iron stores | Normal/↑ (locked) | Normal | ↑ (ring sideroblasts) |
| Serum iron | ↓ | Normal | ↑ |
| TIBC | ↓ | Normal | Normal/↓ |
Pearl: Disproportionately low MCV with normal iron studies suggests thalassemia trait, not ACD.
Take‑home summary
Think hepcidin. Inflammation raises ferritin, lowers TIBC, and traps iron in storage. CKD adds EPO deficiency to the mix. Identify the driver, don’t reflex to oral iron, and use ESAs/IV iron selectively. With practice, the lab pattern practically diagnoses itself.
Step 1 integration recap: IL‑6 → hepcidin ↑ → ferroportin ↓; ferritin behaves as an acute‑phase reactant; CKD → EPO ↓; iron therapy helps only when true deficiency coexists.
Thanks for stopping by! Be sure to check out other blog posts or sign up for tutoring today!